Preparation of Carbon Dioxide
Student Laboratory Experiments with Carbon Dioxide
1. Traditional
limewater test for carbon dioxide
2. Acidity of carbon dioxide
3. Carbon dioxide extinguishes fires
4. Carbon dioxide and aqueous sodium hydroxide react
2. Acidity of carbon dioxide
3. Carbon dioxide extinguishes fires
4. Carbon dioxide and aqueous sodium hydroxide react
Demonstration or Advanced Experiment with Carbon Dioxide
5. Carbon
dioxide/carbonic acid equilibrium
Experiments with Hydrogen
Preparation of Hydrogen
Student Laboratory Experiments with Hydrogen
1. Traditional test for hydrogen
2. Hydrogen forms explosive mixtures with air
3. Reversible conversion of copper metal and copper(II) oxide
4. Reduction of iron(III) oxide with hydrogen
2. Hydrogen forms explosive mixtures with air
3. Reversible conversion of copper metal and copper(II) oxide
4. Reduction of iron(III) oxide with hydrogen
Demonstrations and Advanced Experiments with Hydrogen
5. Effusion of hydrogen is faster than air
6. Hydrogen burns with a gentle flame
7. Disappearing/reappearing candle flame
8. Calcium and calcium hydride produce hydrogen in reactions with water
6. Hydrogen burns with a gentle flame
7. Disappearing/reappearing candle flame
8. Calcium and calcium hydride produce hydrogen in reactions with water
Experiments with Oxygen
Preparation of oxygen
Student Laboratory Experiments with Oxygen
1. Traditional test for oxygen
2. Oxygen supports combustion
3. Dynamite soap
4. Hydrogen-oxygen rockets
2. Oxygen supports combustion
3. Dynamite soap
4. Hydrogen-oxygen rockets
Demonstrations and Advanced Experiments with Oxygen
5. Steel wool burns in oxygen
6. The Blue Bottle experiment
7. Oxygen makes the flame hotter
8. Mini-sponge shooter
9. Chemiluminescence
6. The Blue Bottle experiment
7. Oxygen makes the flame hotter
8. Mini-sponge shooter
9. Chemiluminescence
In our section titled “Lab Experiments”, we provide eight experiments that can be used as full-laboratory period experiments that are useful in teaching specific topics in chemistry. Those marked with * also can be used as classroom demonstrations.
Microscale formation of iron lab*
Microscale reaction between copper(II) oxide and hydrogen*
Mystery Gas
Carbonated Beverages — Priestley’s Soda-water
Molar Mass*
Limiting Reagent
Barometric Pressure without a Barometer
Microscale reaction between copper(II) oxide and hydrogen*
Mystery Gas
Carbonated Beverages — Priestley’s Soda-water
Molar Mass*
Limiting Reagent
Barometric Pressure without a Barometer
Teacher goals for first year should include: (a) incorporate the three gases, carbon dioxide, hydrogen and oxygen into the laboratory curriculum and classroom demonstration repertoire, and (b) implementation of a few of the laboratory experiments listed above.
Some classroom demos

Carbon dioxide is a weak acid and slowly converts blue Universal indicator starting at pH 8 to a mildly acidic solution (yellow).

Hydrogen/oxygen rocket (plastic pipet bulb) launched in the dark

Carbon dioxide is a weak acid and slowly converts blue Universal indicator starting at pH 8 to a mildly acidic solution (yellow).

Oxygen generated in a
syringe and transferred to a large test tube. Steel wool, held
with a metal hemostat is ignited with a 9-v battery and plunged into
the oxygen

Document camera or
overhead projector demo: Nitrogen dioxide is injected to a sealed food
storage bag containing a well plate filled with water as models of
lakes. Some of the lakes are buffered.
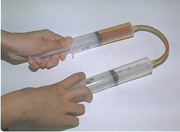
Oxygen is added to
colorless nitrogen monoxide (formerly in top syringe) to give the
instantaneous formation of reddish nitrogen dioxide.

Hydrogen/oxygen rocket (plastic pipet bulb) launched in the dark

Chemiluminescence with oxygen