Study of the iodide reaction with ozone works well in this trial shown here. The solution of KI is starting to form aqueous I2. We use a solution that is 0.0010 M KI and 0.10 M H2SO4.

Yellow iodine appears after just a few minutes.

We next add starch and titrate with standardized thiosulfate solution.
31 May 2002
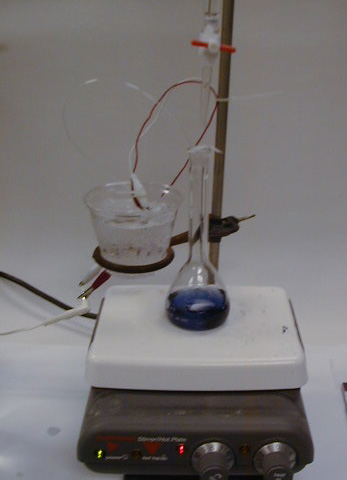
Here we are getting ready for the reaction between
ozone
and KI(aq). (Tere page 13) The ozone generator utilizes Pt and
grapite
and 3 M H2SO4(aq)
in a Beral pipette. The stem of the pipet is drawn out to make a
delivery tube about 30 cm in length. Ozone generator is cooled in an
ice
bath. Here you can see that we are testing for bubbles while
purging
the air from the generator.

Here moist starch-iodide indicator paper detects
ozone.

A view of the bubbles being generated.

We added a drop starch to the solution to detect
iodine.
We then titrate with thiosulfate until the blue disappears. We
titrated
every 10 minutes and could do so without stopping the ozone
generator.
As you can see here, the ozone generator is still delivering ozone to
the
flask.
me titrating...

The results:

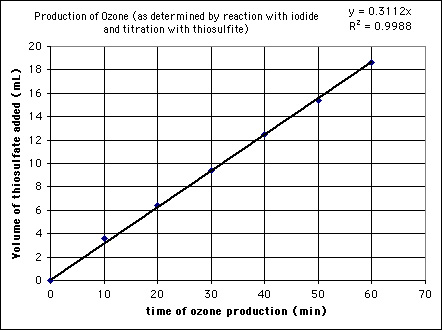
Tere has repeated the experiment for a period of 2
hours
and the rate of ozone generation remains constant. As you can see
from the calculations below, we are making 785 nanomoles ozone per
minute
and the gas produced is 0.25% O3
and (presumably) 99.75% O2:

Preparation of Potassium Iodate Solution. This solution is used for standardization of sodium thiosulfate solution. Exactly 0.0883 g of KIO3 (analytical reagent) was dissolved in water to make 100.00 mL solution. Given the molar mass of KIO3 to be 214.0 g/mol, the concentration of this solution is exactly 4.13 x 10-3 molar.
Preparation of 0.005 M Sodium Thiosulfate Solution. A solution of sodium thiosulfate with an approximate molarity of 0.005 mol/L was prepared by dissolving 1.24 g sodium thiosulfate pentahydrate, Na2S2O3.5H2O (FM = 248.18) in water to make 1.000 L.
Titration of Sodium Thiosulfate Solution. (Bruce) Exactly 10.0 mL of the 4.13 x 10-3 M KIO3 solution was titrated with the approximate 0.005 M Na2S2O3 solution using several drops of a starch indicator solution. The reaction is:
6 H+ + IO3- + 6 S2O3-2 ---> I- + 3 S4O6-2 + 3 H2O
The titration (until the blue colour of I3-/starch disappeared) took 49.0 mL of the sodium thiosulfate solution. Given that 4.13 x 10-5 mol KIO3 was used and the above reaction requires 6 mol thiosulfate for each 1 mol iodate, the 49.0 mL of sodium thiosulfate solution contained 6 x 4.13 x 10-5 mol = 2.48 x 10-5 mol sodium thiosulfate. Thus, the concentration of the sodium thiosulfate solution is calculated to be 5.06 x 10-3 M.
We also prepared a dilute solution that was 5.06 x 10-4 M for use in some experiements.
Here are Tere's results with the Pb/C electrodes. (Tere page 16) Her rate of ozone generation is 14% larger than with the Pt electrode (897 nanomoles ozone per minute). Given that the rate of gas generation was 9.0 mL/minute, the percent ozone was about the same as with Pt. 0.24%.







